Researchers from the Parkinson’s Institute and Clinical Center (PICC) participated in the largest therapeutic trial ever conducted in essential tremor (ET) called PROspective study for SymPtomatic relief of Essential tremor with Cala Therapy (PROSPECT). The landmark study found that nerve stimulation with Cala Therapy can significantly reduce hand tremors. Topline results from PROSPECT were presented today in a late-breaking poster presentation at the International Congress of Parkinson’s Disease and Movement Disorders in Nice, France.
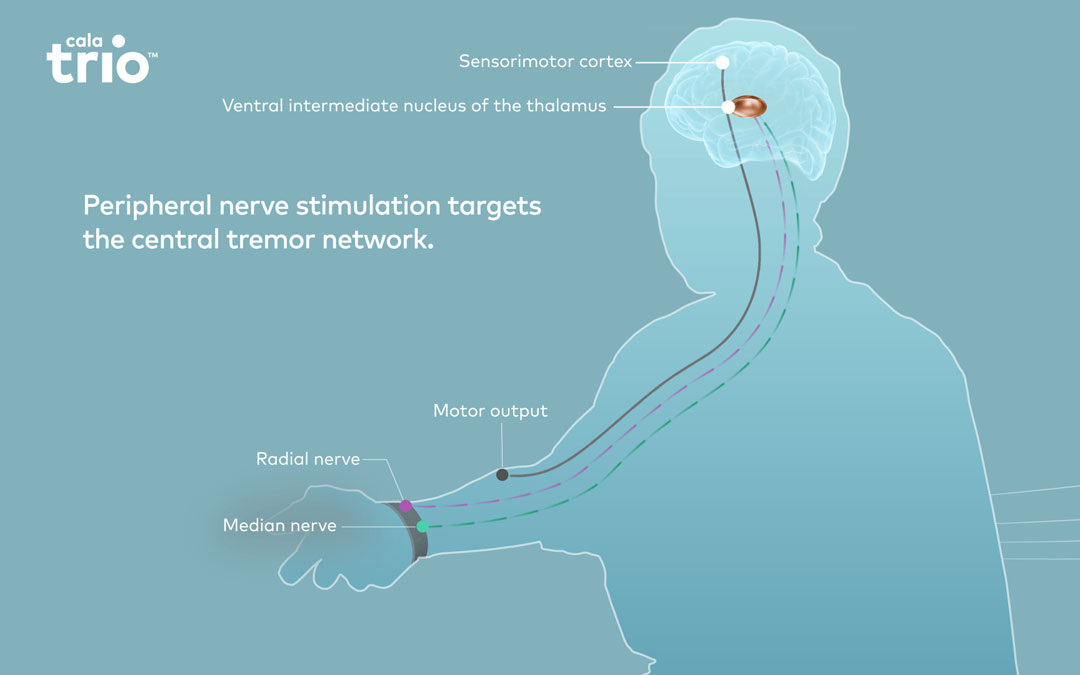
Cala Therapy, now marketed as Cala TrioTM, is a wrist-worn neuromodulation therapy that provides symptomatic relief of ET in the hand. ET is the most prevalent tremor disorder and one of the most common neurological disorders affecting approximately 7 million people in the United States. It can affect almost any part of the body, but the trembling most often occurs in the hands, making everyday activities such as eating, writing, or getting dressed extremely difficult. Current ET treatments include mostly off-label use of medications or brain surgery.
“We are proud to be at the forefront of ET research and part of the largest therapeutic trial in this chronic and progressive disease,” said Dr. Christopher Way, D.O., lead investigator of PROSPECT at PICC. “Non-invasive treatment with Cala Trio provides patients with a safe and effective therapeutic option to surgery or drugs. We are pleased to be one of the select locations in the country to make it available to patients by prescription.”
The PROSPECT study met its co-primary and secondary endpoints, achieving statistically significant improvements in symptomatic relief of ET in the treated hand at three months compared to baseline. The improvements were measured using The Essential Tremor Rating Assessment Scale (TETRAS), a physician rating, (p< 0.0001) and Bain & Findley Activities of Daily Living (ADLs), a patient rating, (p<0.0001). Tremor power was measured using motion sensors (p< 0.0001). No device-related serious adverse events were reported.
“We are thrilled with the results of the PROSPECT trial. It is tremendously exciting to see the relief our non-invasive neuromodulation therapy brings to patients with ET,” said Kate Rosenbluth, Founder and Chief Scientific Officer at Cala Health. “We are deeply grateful to the patients and investigators at the Parkinson’s Institute and Clinical Center who participated in this study.”
Details about the PROSPECT Study and Topline Data
PICC participated in PROSPECT, a prospective, 26-center, single-arm study designed to evaluate the symptomatic relief from hand tremor in ET with repeated use of Cala Therapy. Patients were instructed to use the device for 40-minute sessions twice daily for three months. The device was calibrated to each patient’s tremor frequency and delivered patterned electrical stimulation to nerves through the skin. The study enrolled 263 patients with an average duration of ET symptoms lasting over 25 years.
Data show 62% of patients improved in tremor severity from severe/moderate to mild/slight according to TETRAS and 68% of patients improved in tremor severity from severe/moderate to mild according to ADLs (baseline vs. three months). The average symptom relief lasted 96.7 + 12 minutes after each stimulation session for patients that reported benefit.
Secondary endpoint analysis of motion sensor data obtained by Cala Therapy showed that 54% of patients experienced greater than 50% improvement in tremor power for the three-month study. Over 21,000 stimulation sessions were collected and analyzed. Clinical and patient global impression of improvement showed that 68% of clinicians and 60% of patients rated improvement at three months. Significant improvement was also reported in Quality of Life in Essential Tremor Questionnaire (QUEST) scores. Transient device-related adverse events such as wrist discomfort, skin irritation, or pain occurred in 18% of patients. None required medical intervention.
About Essential Tremor (ET)
ET is the most prevalent tremor disorder and one of the most common neurological disorders, affecting an estimated seven million people in the United States. It is a chronic condition that causes involuntary and rhythmic shaking and typically worsens over time. ET can affect almost any part of the body, but the trembling most often occurs in the hands, making everyday activities such as eating, writing, or getting dressed extremely difficult. ET is often confused with Parkinson’s disease, although it is eight times more common. A key difference is that hand tremors caused by essential tremor happen with goal-directed movement (with intention) whereas Parkinson’s disease tremors occur mostly at rest. Current ET treatments include mostly off-label use of medications or brain surgery.
About Cala TrioTM
Cala Trio is a breakthrough on-demand therapy for hand tremor in adults with essential tremor (ET). The wrist-worn neuromodulation device is the only non-invasive individualized therapy for ET. Cala Trio is calibrated to each patient’s hand tremor. When activated, the device gently stimulates the nerves responsible for the tremor to provide symptomatic relief. Cala Trio is FDA cleared and currently available by prescription in select US markets. For more information, visit www.CalaTrio.com.
About Cala Health
Cala Health is a bioelectronic medicine company transforming the standard of care for chronic disease. The company’s wearable neuromodulation therapies merge innovations in neuroscience and technology to deliver individualized peripheral nerve stimulation. Cala Health’s lead product, Cala TrioTM, is the only non-invasive prescription therapy for essential tremor and is now available through a unique digital business model of direct-to-patient solutions. New therapies are under development in neurology, cardiology, and psychiatry. The company is headquartered in the San Francisco Bay Area and backed by leading investors in both healthcare and technology. For more information, visit www.CalaHealth.com.